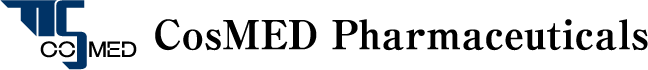Research & Development
Overview of TTS Technology
CosMED Pharmaceutical researches, develops and manufactures products relating to “a system to safely and efficiently deliver drugs through skin” in an innovative way on the basis of medical adhesive technology, gelatinization technology, transdermal absorption promotion technology and skin penetration evaluation technology, all of which are important elemental technologies for TTS (transdermal therapeutic system), and based on deep knowledge of skin and transdermal drug absorption that have been cultivated from the basic research of transdermal absorption in Kyoto Pharmaceutical University.
As the result of our multidirectional approach to “transdermal absorption”, we have succeeded in the development of microneedle technology to make hyaluronic acid and collagen into pinholder-like acicular crystals by ultrafine processing technique and practical realization of the world’s first cosmetics with microneedles. The technology is expected to be applied in medical field as the next generation TTS to deliver macromolecular drugs directly into skin only by patching on skin.
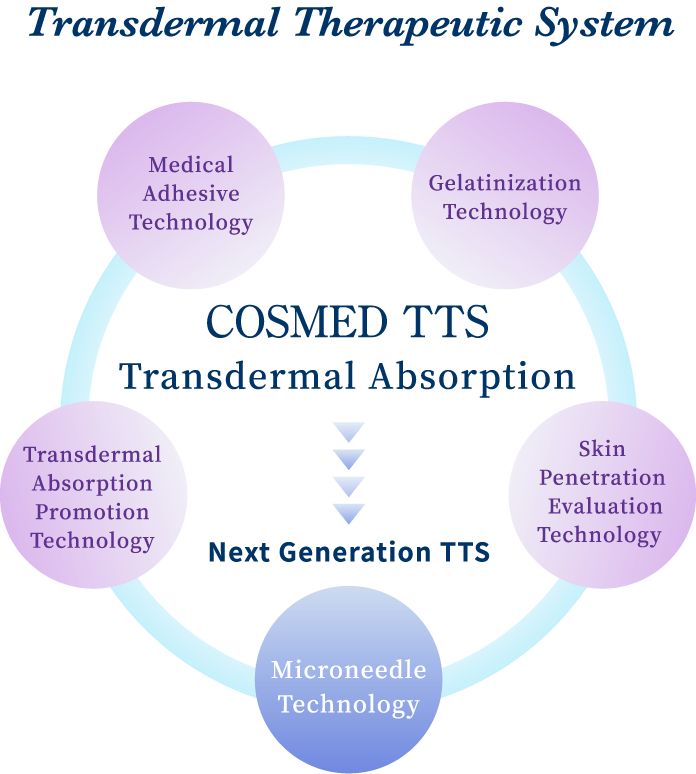
[Next Generation TTS] Microneedle Technology
The microneedle technology is a new technology representing our TTS technology to enable safe medical treatment putting less burden on patients by controlling dosage and reducing side effects. It is an innovative technology that greatly contributes to the future development of medical treatment. We are the pioneer of productized soluble microneedles and have accumulated a large amount of know-how on design and quality control to meet users’ needs in development and manufacture of products.

Medical Adhesive Technology
Medical adhesive technology is a combined technology of medical adhesives to patch drugs on skin for medical treatment. Medical adhesives should meet various requirements, such as less of a burden on the skin, adhesiveness not influenced by site of skin, health condition of patients and weather, controllable patching time, etc. and thus only a specialized organization understanding transdermal absorption system can provide optimum adhesives by the technology.

Gelatinization Technology
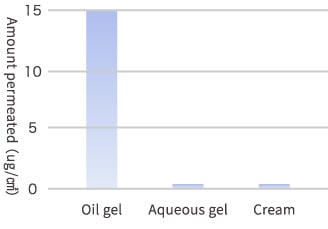
The gelatinization technology is used to prepare a gel by dissolving a plasticizer with macromolecular polymers that have already been employed for skin application. The typical oil gel prepared by impregnating a skin-friendly polymer material with large volume of natural vegetable oil or mineral oil is a new functional base component for drugs and skin care products. The oil gel does not temporarily deliver moisture directly to skin being different from aqueous gel but surely make the active ingredient in the gel penetrate into skin while firmly retaining the moisture of skin. The oil gel has been studied and developed by the specialists of transdermal drug absorption. The indispensable nutrients for skin, such as vitamins A, C and E, contained in oil gel penetrate into skin to an amount that is hundreds times higher than that of the nutrients in aqueous base components (aqueous gels, creams, lotions and emulsions).

Transdermal Absorption Promotion Technology
We are studying to realize a transdermal absorptive formulation that delivers active ingredients more safely and efficiently by using the technology of preparing adhesive polymer oil gel and transdermal absorption promoters that have already been employed as drug additives.
Skin Penetration Evaluation Technology
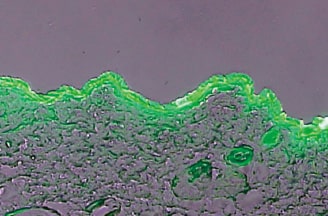
Skin penetration of the active ingredients of cosmetics and drugs is evaluated by the TTS research devices developed in-house. The devices enable the evaluation using various skin types including artificial skin, cultured skin and human skin, measurement of the amount of combined ingredients penetrating into stratum corneum, epidermal layer and dermis layer and the amount of transdermal permeation of the ingredients, and visual evaluation of skin penetration behavior of the ingredients with photomicrographs.

Production System
Each product is produced in Japan with care to ensure safety
The products of CosMED Pharmaceutical are productized domestically from the research and development stages to manufacturing stage under in-house management. The products of CosMED Pharmaceutical, not only drugs but also cosmetics, are manufactured in a manufacturing environment meeting the quality control standard similar to that for manufacturing drugs.

“The Second Katsura Factory (Kyoto City)” (our own factory) was opened in 2017,
“The Third Kissho Factory (Kyoto City)” (our own factory) was opened in 2020.
Products are manufactured in the highest order clean rooms complying with ISO22716 (Cosmetic GMP*) and each product is carefully handled throughout the entire process from manufacturing to packaging to ensure high quality and constant supply under strict quality control meeting the standard for manufacturing drugs.
We streamline production lines at the head office factory, Katsura factory, and Kissho factory.
In addition , We will also increase production capacity and further strengthen our supply system.


| Cosmetic Manufacturing License | Cosmetic Manufacturing & Distribution License | Quasi-drug Manufacturing License | Quasi-drug Manufacturing & Distribution License | Certificate of registration for medical device manufacturer |